People
Principal Investigator
Research Assistant
-
Alvaro Larrañaga San Miguel
Latest publications
-
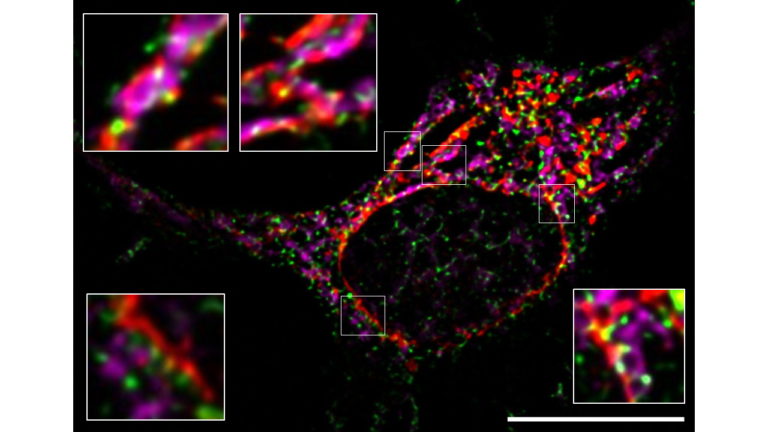
Modulation of Mitochondria–Endoplasmic Reticulum Contacts (MERCs) by Small Molecules as a New Strategy for Restoring Lipid Metabolism in an Amyotrophic Lateral Sclerosis Model
Journal of Medicinal Chemistry (Jan, 2025) DOI: 10.1021/acs.jmedchem.4c01368 -
Crosstalk between mitochondria–ER contact sites and the apoptotic machinery as a novel health meter
Trends in Cell Biology (Jan, 2025) DOI: 10.1016/j.tcb.2024.08.007 -
Targeting mitochondrial metabolism by the mitotoxin bromoxib in leukemia and lymphoma cells
Cell Communication and Signaling (Nov, 2024) DOI: 10.1186/s12964-024-01913-2 -
Novel meriolin derivatives activate the mitochondrial apoptosis pathway in the presence of antiapoptotic Bcl-2
Cell Death Discovery (Mar, 2024) DOI: 10.1038/s41420-024-01901-y