Amyloid-β disrupts oligodendrocyte function and myelination
New insights from zebrafish research reveal how amyloid-β affects early myelination through PKC signaling
A growing body of evidence is reshaping how glial cells are viewed in the context of neurodegenerative diseases. No longer seen as passive support cells, oligodendrocytes are emerging as active contributors to central nervous system pathology.
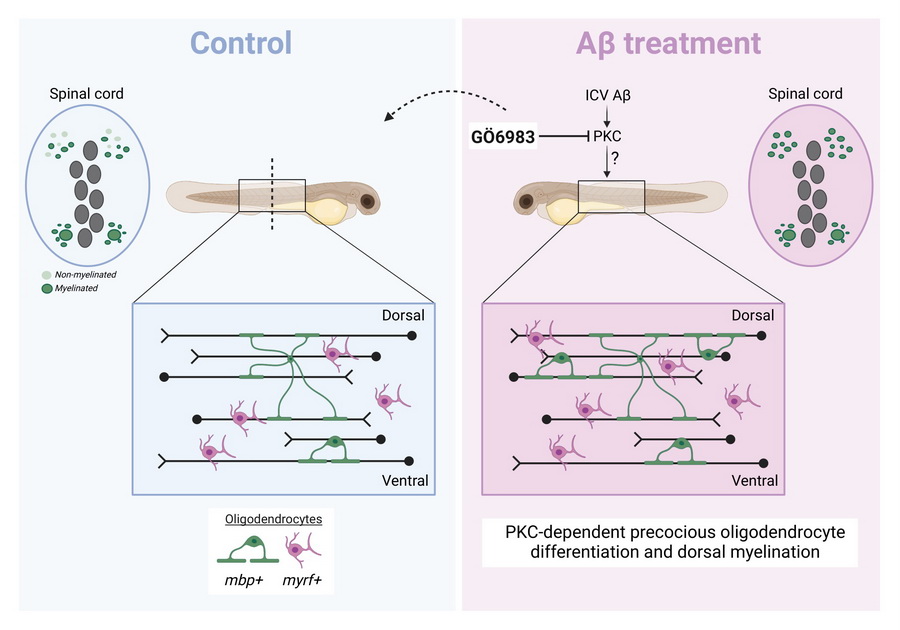
A recent study by researchers from the Gliomatrix lab at ACHUCARRO and the University of the Basque Country (UPV/EHU) reveals that amyloid-β (Aβ), a hallmark of Alzheimer’s disease, disrupts oligodendrocyte lineage dynamics and impairs myelination through a protein kinase C (PKC)-dependent mechanism.
In collaboration with the Appel Lab at the University of Colorado Anschutz Medical Campus, the team used zebrafish as a live in vivo model to visualize oligodendrocyte development and myelin formation. Their results show that Aβ exposure induces precocious oligodendrocyte differentiation and aberrant myelin sheath formation, driven by PKC activation. Importantly, pharmacological inhibition of PKC successfully restored normal oligodendrocyte development and myelination, highlighting PKC as a potential therapeutic target.
These findings challenge the traditional neuron-centric perspective of Alzheimer’s disease, positioning glial dysfunction —particularly oligodendrocyte impairment and white matter disruption— as early and critical events in disease progression.
This work underscores the importance of broadening Alzheimer’s research to include glial cells as central players in neurodegeneration, opening new avenues for early intervention and treatment.
Read the full paper here:
